What does Contract Research organization stand for in pharmaceuticals?
CROs in Pharmaceuticals

A CRO – Contract Research organization is a company that conducts complex medical research. This is often do to reduce the costs of the research process. Instead of maintaining office space and other infrastructure, the drug company receives a team of medical experts that conducts the testing.
This method saves the drug company time and money. It has become more common for drug companies to outsource their medical testing responsibilities to a Contract Research organization. Besides cutting the cost of research, drug companies want to eliminate the ongoing cost of maintaining a full-time staff and medical facilities.
Contract Research organization – CRO
As the number of pharmaceuticals in the market grows, Contract Research organization services are becoming increasingly most important for Pharma companies. The rise of globalization has spur research and development, creating new job opportunities.
In the pharmaceutical sector, increasing focus on the global health crisis has led to increased costs and time spent on clinical trials. CROs face challenges in retaining competent experts, primarily due to high competition from pharmaceutical, biotechnology, and medical device companies.
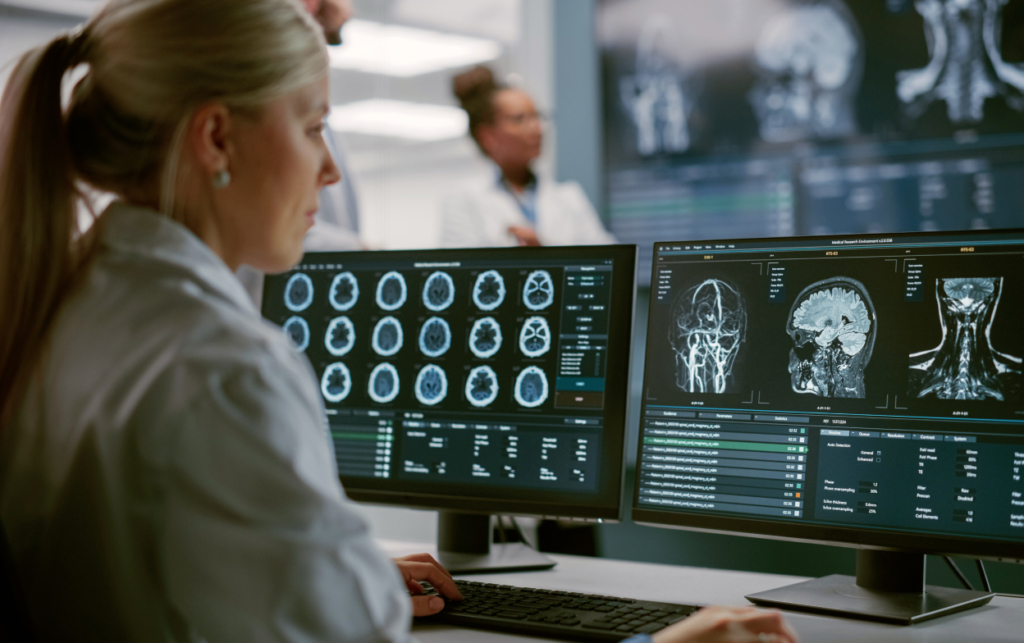
As a result
They have to offer higher salaries to attract and retain highly skill workers. Increasingly, pharmaceutical and biotech companies are using CROs to streamline clinical trial processes and cut costs. Also, These organizations help pharmaceutical sponsors reduce costs by implementing and managing complex medical research without having to invest in office space and infrastructure.
Also, They provide expertise in the medical field, thereby saving time and resources. CROs are becoming increasingly most important for Pharma companies, as they seek to reduce their costs and the ongoing expense of medical facilities and full-time staff.
Working with a CRO provides hiring companies with access to advance technology, systems, and data management. Clinical research is rapidly changing, and IT capabilities and internet-base applications are essential for speeding up clinical trials and ensuring quality control. It is most important to look for a CRO that has a prove track record for quality assurance and quality control.
Increasing globalization and cross-border regulatory processes have blur location boundaries. This makes it critical to work as a team to materialize ideas and products.
In this environment
Drug/device makers and contract research organizations (CROs) play a critical role in the chain of discovery. They develop and validate products in preclinical experiments and then take them to the market. Project management teams are responsible for nurturing the materialization process in a collaborative manner.
CROs are private companies that perform clinical research functions for sponsors. They are playing a more prominent role in pharmaceutical clinical trials. They often interact with clinical investigators and respond to their queries. This is especially most important in medical oncology, where there are numerous investigational compounds and ongoing interventional trials.
Vial CRO
Vial CRO is a global bio-pharmaceutical solutions organization with an impressive 22-year track record. The company offers the complete range of clinical, commercial, and supply-chain services. The company’s operating model is unique and its experience and industry relationships are unmatch. The company is growing at an impressive CAGR of 6.8% for its CRO business and is targeting a 13-14% EBITDA margin by FYE 2020.
In order to continue to build a more comprehensive offering, Vial CRO is making key changes. The company has promote Michelle Keefe to CEO from her previous role as president of medical affairs and commercial solutions. She replaces Alistair MacDonald, who is leaving the company after more than two decades.
He will serve as an advisor until March 2023
Keefe is a highly experience healthcare executive with over three decades of experience. The organization also appoint Michael Brooks to the role of chief operating officer. Brooks previously serve as global head of clinical development solutions.
Read also: Your Back Will Thank You For Reading This Article
Vial CRO focuses on the clinical, regulatory, and quality aspects of the pharmaceutical process. It offers a full suite of advisory services that integrate the research process into the overall strategy of the pharmaceutical client. Its research portfolio is diverse, ranging from small Pharma to big Pharma. It has cultivate long-standing relationships with clinical research sites and principal investigators. This helps create consistency across expensive clinical trials.
Vial CRO update its FYE2019 guidance to June 2020, and it shows a modest decline in Q1. The company’s FYE2020 guidance calls for a marginal increase in earnings, but the company’s revenue growth is expect to remain in the range of five to eight percent. Depending on the size of the new Pfizer contract, Vial CRO may post a positive to neutral growth in FYE2020. The company has an ongoing program to increase its EBITDA margins call Forward-bound.
In addition to offering job-specific training and certification, Vial CRO offers many different career paths. As an employee, you’ll have the opportunity to develop new intellectual property and lead a team. As a contract research organization, you’ll also have the chance to work with leading Pharma and biotech companies.

Medpace
Medpace is a mid-size contract research organization (CRO) base in Cincinnati, Ohio. It provides pharmaceutical, medical device, and regulatory services to clients around the world. It employs approximately 3,600 people in 37 countries. Although large CRO companies still account for the majority of the CRO market, smaller and medium-size organizations have begin to gain share and have become preferred partners for pharmaceutical companies.
The size of Medpace is an advantage, as it retains a cohesive office culture. Most other companies have a largely home-base culture, which can have a negative impact on employee morale and client interaction. Additionally, Medpace’s size allows it to partner with clients in an innovative way.
Found in 1987
Medpace is one of the largest pharmaceutical CROs in the world. It claims to have the largest central laboratory network. It is a certify equal opportunity employer and has been recognize as one of the top LGBTQ + workplaces by the Human Rights Campaign organization. Its 70,000 employees support clinical research efforts in nearly 100 countries worldwide.
Medpace’s team members have extensive clinical and regulatory experience and possess fluent multi-lingual skills. Base in the Netherlands, the company also has offices in Poland, Israel, and the Czech Republic. Its cross-cultural expertise helps the company to offer clients a full range of services, including regulatory, clinical, and quality guidance.
Medpace is consistently rank as a top CRO in pharmaceuticals by Igea Hub. The company develops life-saving and life-improving drugs through its contract research and development activities. It also provides regulatory consulting and statistical analysis to its clients. In August 2017, it acquire symphony health for $530 million. In 2018, Medpace had revenues of $2.87 billion.
Medpace is one of the largest CROs in the world. Its services encompass a variety of therapeutic areas, including cardio-vascular, oncology, rare diseases, and central nervous system. Medpace employees have extensive experience in a broad range of therapeutic areas. Medpace`s international presence enables it to conduct clinical trials in a number of countries worldwide.
CTI Clinical Trial and Consulting Services
Dynakin is a contract research organization with a GLP bio lab that provides model-drive drug development services. Founded in 1997, Dynakin has nearly two decades of experience and strong local expertise across Europe. CTI acquired Dynakin to grow its European capabilities. The companies share a similar culture and philosophies, including a commitment to employee retention and career development.
CTI is a privately held global CRO with a global reach. Also, It specializes in rare diseases, stem cells, and cell and gene therapies. It also provides comprehensive research services for pharmaceutical companies and operates in North America, Europe, Latin America, Asia-Pacific, and the Middle East/Africa region.